Life Sciences organizations are finding new ways to work within distributed and hybrid work processes adopted during the pandemic that remains in place today. Mastering modern work requires secure and compliant management of regulatory content—whether your OpenText™ Documentum™ Content Management (CM) for Life Sciences repository is off-cloud, hosted in your private cloud or hosted by OpenText in OpenText™ Content Cloud for Life Sciences.
The latest announcement by OpenText of Cloud Editions (CE) includes some great updates to OpenText™ Life Sciences solutions. Check out the latest updates below.
January 2025: What’s new in OpenText Documentum Content Management for Life Sciences CE 24.4
The OpenText™ Documentum™ Content Management (CM) for Life Sciences CE 24.4 release includes new capabilities designed to help organizations simplify upgrades, increase ease of use, supercharge indexing, streamline and automate tasks, and expand use to and global workforce.
Cross-portfolio new feature benefits
Gain insights to help manage current and future investments with new license management and tracking functionality
The new license management, powered by the Software Protection Services module of OpenText Directory Services (OTDS), provides tracking functionality that makes it easier to track and allocate software resources and provides insights into software usage patterns so that you can make informed decisions about current and future investments.
Supercharge your indexing with highspeed search and indexing capabilities
Documentum Search is now certified with OpenText Documentum CM for Life Sciences and is the default search engine for all deployments. Documentum Search completes indexes with improved throughputs in indexing larger number of documents and larger data size in given time.
Overcome information silos and leverage external systems with confidence using the new integration framework
An integration framework is introduced in this release for better integrations with external applications with proper authentication and authorization. This framework provides a configurable mechanism to make REST API calls run as a job or run during a lifecycle transition. It also enables integrators with configurable processing for sending or reading metadata to external systems.
Stay compliant with new group audit and user audit functionality that helps demonstrate segregation of duties and supports user reviews
Group Audit and User Audit query forms help to demonstrate segregation of duties and supports periodic user reviews to show current and previous access roles and permissions.
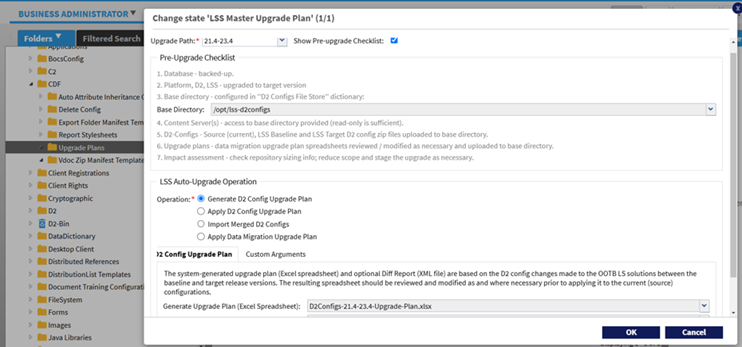
Enhanced Intelligent Upgrade to support additional custom configurations and extension to Smart View.
Intelligent Upgrade is further enhanced in this release to include low-level comparison and merging of D2 menu configurations extending to include Smart View client. Additional enhancements include supporting of new alias columns, expanding the Hybrid classified configurations, and preserving of D2 auto-naming sequence counter values.
Empower privileged users with modern capabilities without compromising current versions
Privileged users will be able to update certain properties of approved Content Templates without affecting template state or version with this release using Restricted Edit.
Reduce deployment time, costs and human error while also meeting compliance requirements and stopping bad actors using the Vault infrastructure
Automating password generation, management, and integration into cloud deployments using the Vault infrastructure centralizes secrets management, providing another layer that ensure sensitive credentials are securely stored, accessed, and rotated, meeting compliance requirements such as SOC2 standards. Vault integration allows OpenText Documentum to participate in TCO reduction across the Enterprise in a compliant manner using approved, centralized/standardized security practices.
Simplified cloud deployment with a single Helm chart, and standardized technologies such as Oracle database and PostgreSQL, provide faster time-to-value
The Life Sciences solution suite is available as pre-built docker images. Certification of Oracle database in addition to PostgreSQL provides options to choose from to align with Enterprise architecture. Support for a single Helm chart provides a streamlined deployment process, including OpenText Documentum CM reducing time-to-value for the solution.
Quality and Manufacturing new feature benefits
Reduce data entry duplication with metadata push/synch capabilities
Using the Integration framework, this release provides the ability to push/sync data from external systems to Documentum CM for Life Sciences. This release includes components for the Documentum for Quality and Manufacturing GMP Material metadata sync such as an external ERP system.
Modern Life Sciences user interface encourages user efficiency using the new Life Sciences Smart View either on-premise or in the cloud
This release includes the new Life Sciences Smart View that can be co-located with the Life Sciences solution either on-premises or in the cloud. This modern UI leverages Documentum Smart View to enable Life Sciences users to perform their day-to-day operations efficiently. Life Sciences Smart View is certified with Documentum for Quality and Manufacturing for Reader, Reviewer, and Approver roles and additionally provides Business Administration capabilities.
eTMF new feature benefits
Expanded language support extends use to a global workforce
Documentum for eTMF is now localized and available in Simplified Chinese and French.
Regulatory new feature benefits
Manage the entire regulatory lifecycle effectively with end-to-end regulatory submission content planning, management, submission and archiving
Documentum for Research and Development and Documentum for Submission Store and View are packaged and licensed together as Documentum for Regulatory starting this release enabling customers to benefit the end-to-end value of Regulatory submission content planning, management, submission, and archiving. Customers gain access to both modules with the flexibility to implement either one or both.
Streamline and automate critical regulatory submission tasks
Documentum for Research and Development artifact dictionary key mappings are updated across all seven domains. This helps with the automatic assignment of artifacts to the appropriate eCTD section. Submission planning and assembly enhancements include submission VDTS enhancements to support a simplified submission plan approval process, plan archiving, and auto-harvesting of draft and approved documents upon VDoc generation. Automatic linking of placeholders for a submission is enabled for effective planning and tracking. This release enhanced the submission plan lifecycles for better management of the plan and hand-off to a publishing solution like Lorenz docuBridge. These updates help organizations enhance their overall regulatory submission lifecycle and help reduce time-to-market.
Expanded language support extends use to a global workforce
Documentum for Research and Development is now localized and available in Simplified Chinese and French.
Expanded M1 VDTS planning template support for Australia, Canada and China
This release expands the M1 VDTS planning template support for the following regions – Australia, Canada, and China. Module 1 VDTS templates can be restricted to a defined region, making it easier to choose the most appropriate template.
China-based submission support
This release introduces artifacts to support the planning and creation of artifacts according to the requirements defined in the China Module 1 Schema version 1.0.
For a complete list of new features and fixed issues, refer to the OpenText Documentum CM for Life Sciences CE 24.4 release notes.
February 2023: What’s new in OpenText Content Cloud for Life Sciences CE 23.1
Content Cloud for Life Sciences meets the demands of work environments for Life Sciences organizations with important enhancements to drive compliance and master modern work.
With CE 23.1, Content Cloud for Life Sciences adds Amazon Web Services (AWS) support to its productivity and governance capabilities of OpenText Documentum for Life Sciences. This allows life sciences companies to optimize their operational efficiencies with AWS while experiencing the fully managed service benefits of predictable operating costs, flexible upgrade timing, and secure employee and partner access.
Content Cloud for Life Sciences helps pharmaceutical companies accelerate clinical trials, improve regulatory submission quality and ensure manufacturing process compliance.
- Choose your technology: Pick the hyperscaler that meets your enterprise needs and delivers on business benefits. The CE 23.1 release adds AWS support, bringing Amazon’s reliability, security and performance to Content Cloud for Life Sciences.
- Work smarter: Lean on a fully managed cloud solution to offload the IT management burden, focusing resources on other key business objectives while benefiting from more predictable operating costs. Flexible upgrade timing ensures the latest versions are always available and are rolled out at a time convenient to the business.
- Move faster: Rely on one repository to share and reuse content for a single source of truth to support collaboration for research, clinical, regulatory and manufacturing processes, with audit-ready confidence and GXP compliance.

For more information about new features and enhancements available in Content Cloud for Life Sciences CE 23.1, check out the release notes, administration guide and validation summary report using your OpenText My Support login credentials.
September 2022: What’s new in OpenText Documentum for Life Sciences CE 22.3
Documentum for Life Sciences meets the demands of modern work environments for Life Sciences organizations with enhancements across the entire suite of Documentum for Life Sciences product.
OpenText Documentum for Quality & Manufacturing
Documentum for Quality and Manufacturing brings efficiency and reduces waste with enhanced printer management. To further enhance current Good Manufacturing Practices (cGMP) print management, Documentum for Quality & Manufacturing CE 22.3 adds a new layer of security in accessing prints. The new Follow-me print feature maintains the chain of custody required by cGMP and allows users to assign prints to a user instead of a printer. On-demand print capability adds user authentication at the time of printing, enhancing security ensuring only the assigned user retrieves the print and gives convenience for the user to retrieve prints from the nearest printer.

To learn more, read the blog post about secure print controls for pharmaceutical manufacturers.
OpenText Documentum for Research & Development
Identification of Medicinal Products (IDMP) is on the mind of Life Sciences organizations worldwide, especially those operating under the governance of the European Medicines Agency (EMA). ISO IDMP standards establish a consistent way to represent medicinal and pharmaceutical products across geographies. Regional Health Authorities adopting IDMP will have the ability to share data in support of public health to improve pharmacovigilance, regulatory efficiency, and patient access to medicines and information. Documentum for Research & Development CE 22.3 brings efficiency and consistency across systems with dictionary updates to include controlled vocabularies, auto-cascading of IDMP metadata, and efficient IDMP keyword-based searches.

To learn more, read the blog post about how to prepare for IDMP 2.x.
OpenText Documentum for eTMF
Signposts: Clinical trial documents and other artifacts that are required to fulfill clinical trial requirements may be stored in a variety of systems. Signposts provide a way to reference these artifacts in other systems and track them as part of the trial process. In Documentum for eTMF CE 22.3, signposts can be created to reference external artifacts and consolidate them into the trial content. This provides Medicines and Healthcare products Regulatory Agency (MHRA) compliance and ensures accuracy and completeness of the trial master file without having to maintain duplicate copies of these documents in multiple locations.
Locking and archiving trials: Archiving clinical trials prevents any further updates from being made to trial artifacts once the trial is closed. A trial is locked before it is archived to allow the trial documents to be analyzed and to determine if the trial is archivable. With Documentum for eTMF CE 22.3, Clinical Archivists can perform trial locking and archiving at the site and country granularity levels in addition to the trial level that’s already possible in prior versions. This provides Medicines and Healthcare products Regulatory Agency (MHRA) compliance and helps manage Clinical trials in different sites and countries independently.
For more information about new features and enhancements available in Documentum for Life Sciences CE 22.3, watch an on-demand webinar and check out the product documentation and release notes using your OpenText My Support login credentials:
September 2022: What’s new in OpenText Life Sciences Smart View CE 22.3
OpenText™ Life Sciences Smart View continues to empower employees and partners by equipping them with the right tools to maintain compliance while boosting productivity and collaboration among increasingly distributed workforces. CE 22.3 adds support for the routing, review and approval of change requests and documents. Many enhancements are also available with this release to streamline review and approval tasks, expand content creation, and provide new ways to access and act upon content.
Routing change requests for review approval
Category one documents require formal review and approval along with signoff by the Quality Organization (QO). Using Life Sciences Smart View CE 22.3, coordinators can attach the category one documents and route change requests for review and approval. Streamlining the review and approval provides the status of the request and also ensures compliance and audit tracking.

For more information about this feature, read the Product Forum post about routing change requests for review approval using your OpenText My Support login credentials.
Streamlining review and approvals of change requests
Life Sciences Smart View CE 22.3 streamlines the process for completing change request reviews and approvals. On a single screen, approvers have quick access to the change request along with the content and information about the documents in the request. This allows approvers to review the content at the time of approval without having to use any external tools to open the document.

For more information about this feature, read the Product Forum post about the review and approval of change requests.
Routing category one documents for review approval
Once the change request is approved, the affected documents must be routed for review and/or approval. In Life Sciences Smart View CE 22.3, coordinators or authors can easily select the related documents of the approved change request and quickly route them.

For more information about this feature, read the Product Forum post about routing category one documents for review approval.
Revising workflow tasks for more efficiency
During document routing when reviewers or approvers reject tasks, the attachments are automatically demoted to a draft status and are forwarded as a task for respective authors. Life Sciences Smart View now allows authors to revise these documents to reflect provided feedback and resend for another review or final approval. Changes made as part of this process are reflected in revision history of the document, providing a comprehensive audit trail.

After document approval, coordinators specify when the documents become effective along with other review and access settings. As part of coordinator approval, Life Sciences Smart View guides the user through entering effectivity parameters.

By supporting change requests and affected document review and approval tasks using Life Sciences Smart View workflows, all participants save time with immediate and streamlined access to all the information required to do their work.
For more information about this feature, read the Product Forum post about performing document rejection and making effective workflow tasks.
Visit the Life Sciences Smart View Product Forum using your OpenText My Support login credentials where you can:
- Read blog posts
- Look at new feature videos
- Collaborate with product team members and other users
July 2022: What’s new in OpenText Life Sciences Smart View CE 22.2
OpenText™ Life Sciences Smart View continues to empower modern workers with a user experience that improves productivity and drives distributed, agile work processes. CE 22.2 adds support for the full content lifecycle for regulatory virtual document assemblies built with content managed by OpenText™ Documentum for Research & Development.
Assemble and manage virtual documents
Virtual documents are a useful way to re-organize documents into new assemblies across functional areas. Clinical study report components and CTD Modules are good examples for using virtual documents.
Because of their nature, virtual documents are assembled and refined over time. To help with this process, Life Sciences Smart View supports the complete content lifecycle of create, revise, route, review, approve and consume.
Virtual document capabilities include:
- Dynamically create, revise and remove content
- Compare side-by-side with previous versions or other assemblies
- Add documents from another virtual document, from a collection or by browsing or searching the repository.
Using the new compare capability, users save time identifying the differences in virtual document against its previous versions or another virtual document.

Easily add documents
Authors can now easily create and import documents with properties or contents copied from recent and favorite documents while authorized readers can import new documents using the simple wizard-driven process.
Other enhancements that improve productivity include:
- Filter views using effective labels reflecting document status
- Sort and filter browsed content and search results dynamically
- Share user collections with other users
Visit the Life Sciences Smart View Product Forum using your OpenText My Support login credentials where you can:
- Read blog posts
- Look at new feature videos
- Collaborate with product team members and other users
December 2021: What’s new in OpenText Life Sciences Smart View CE 21.4
CE 21.4 brings some great enhancements to OpenText™ Life Sciences Smart View, a SaaS application that gives your employees and authorized partners easy access to your regulatory content anytime, anywhere via their device of choice—whether your OpenText Documentum for Life Sciences repository is off-cloud, hosted in your private cloud or in OpenText™ Content Cloud for Life Sciences. Check out the latest updates below.
External users can approve tasks with e-signature
Pharmaceutical and medical device companies often use contract manufacturing organizations (CMOs) to help boost production capacity. CMOs need their sponsors to approve documents such as methods, specifications, or procedures related to critical manufacturing processes.
The task approval in Life Sciences Smart View allows external users and partners to securely perform approval tasks in the system of record with audited e-signature.

Easily add and view document versions
Content authors can import content created offline as a new version. This is an alternative means for updating documents.

Users can quickly access all versions of a document including their properties and contents. The document status is visually indicated with easily noticeable color coding.
Create medical device content
Authors can now create medical device content using the intuitive wizard-driven process that helps in selecting the appropriate template and adding metadata.

Share collections with others
Collections are related documents, forms, searches and locations grouped together for quick access. Collections can now be shared with other users for improved collaboration. For example:
- Collections containing methods, specifications and batch records can easily be shared externally to contract manufacturers and contract labs, and internally among Manufacturing Associates, Quality Control Analysts and Quality Associates.
- Collections containing stability reports and executed master batch records can be shared with technical writers for quick access to the documents they need.
- Collections containing effective methods can be shared with workers on the manufacturing floor.
- Research & Development authors and managers can share collections of study plans and reports, labeling documents, non-clinical and clinical study protocols with the internal and external stakeholders.

Initiate workflow
Authors and coordinators can route documents for review and approval to bring efficiency to their collaboration and approval processes. This allows users to effectively manage the full lifecycle of content within Life Sciences Smart View, including creation, revision, routing, review, approval, and consumption.

Take a few minutes and learn more about the efficiency and security gains of OpenText Life Sciences Smart View and visit the product forum to watch new feature videos.




